Call us for more information 919.795.9434

186 Pinecrest Road, Princeton, NC, 27569
Are You Prepared?
We Are
Each year brings new change and new laws for regulation and manufacturing of pharmaceuticals. Our team will keep you ahead of the curve as we support your staff and teach them how these changes will affect their current practices.
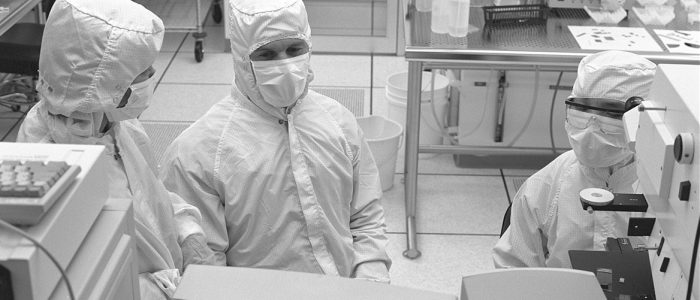
Aseptic process regulations are evolving, requiring sterile manufacturing facilities to change how they process product. National consultants have designed environmental monitoring systems, trained clients on how to maintain the validated state and implemented programs to keep these areas clean and within FDA standards.
NationalPBS has worked with industry leaders





Working As One
Regulatory bodies all use different standards to define a team as GMP certified. In a global market it is essential for a product to be able to transition seamlessly over borders. We spend the time to learn how each regulatory agency defines GMP standards and can make sure your staff is covered on all fronts. Let us come to you and demonstrate how we can set measurable outcomes to ensure compliance.
Making Headlines

As patents expire and the pharmaceutical industry slows its explosive growth, to keep competitive companies have to constantly evolve. We can help take your team into the generic manufacturing market and stay ahead of the competition.